Direct and Indirect Allorecognition
One of the reasons why the HLA system acts as such as efficient immunological barrier to transplantation and transfusion is the fact that HLA molecules can be recognized directly or indirectly by the cells of the immune system.
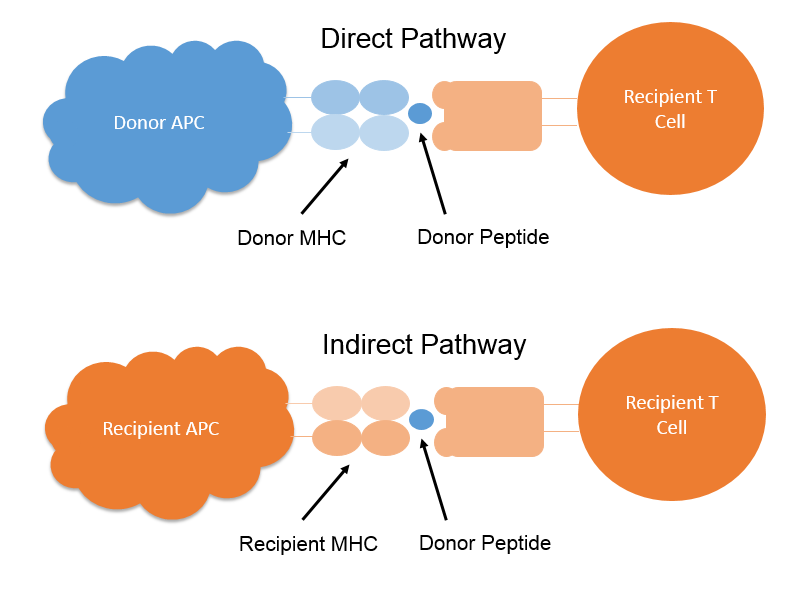
In the Direct Pathway, following kidney transplantation, donor derived dendritic cells, triggered into maturation by the transplant Ischemic and Reperfusion Injury (IRI) as well as locally released pro-inflammatory cytokines, migrate from the kidney to the recipient lymphoid organs where they stimulate the host immune response by presenting host and donor MHC derived peptides loaded into intact donor dendritic cell MHC, to host T cells. This Direct Pathway of allorecognition is the main route for stimulating of the acute phase of the alloimmune response. In the normal immune response, only a small proportion (less than 0.1%) of host T cells will be specific for a given MHC-peptide complex, however in the alloimmune response, a high number of T cells (approximately 10%) react to the allogeneic MHC-peptide complex leading to a strong alloimmune response. Indeed, depletion from the graft of donor dendritic cells prior to transplantation has been shown to lead to graft tolerance or at least a reduction in acute rejection.
In the Indirect Pathway, following kidney transplantation, host dendritic cells which infiltrate the graft as part of the post-transplant inflammatory process have their MHC loaded with donor peptides derived from the take up and processing of donor MHC. Following migration to the lymphoid organs, these MHC-peptide complexes are presented to host T cells. This indirect pathway is similar to the process by which antigen is presented to T cells in the normal immune response and involves a smaller number of T cells than the direct response.
A number of studies have indicated that, in solid organ transplantation, T cell alloimmune response elicited by the Direct Pathway decreases with time post-transplant whilst the T cell alloimmune response elicited via the Indirect Pathway which can also be initiated in the acute phase, sustains and contributes to chronic rejection.
A third pathway the Semi-Direct Pathway of allorecognition has been described in which host antigen presenting cells acquire intact MHC molecules from donor cells thus allowing direct presentation to host T cells. This pathway may contribute to sustaining the alloimmune response.
Stages of Allograft Rejection
Rejection of the renal allograft has been classified into four key stages related to the timing of the rejection – hyperacute rejection, accelerated rejection, acute rejection and chronic. Hyperacute rejection occurs within minutes to hours of transplant. Accelerated rejection occurs within days, acute rejection occurs within days to weeks, while Chronic rejection occurs months to years post-transplant.
Hyperacute rejection occurs almost immediately after the organ is transplanted and is usually due to the presence of pre-formed donor specific anti-HLA antibodies. The mechanism of hyperacute rejection involves deposition of antibodies against HLA antigens expressed on the endothelium (of the glomeruli in the case of kidneys) and the graft microvasculature. This leads to activation of the classical complement cascade causing endothelial necrosis, platelet deposition, local coagulation causing massive thrombosis in the capillaries, which prevents the vascularization of the graft.
Hyperacute rejection is usually accompanied by C4d deposition, though this may be negative early on. Hyperacute rejection is not typically reversible and requires the immediate removal of the graft. Accelerated rejection usually occurs within days and is due to a memory or anamnestic response to pre-formed antibodies directed against mismatched donor antigen which are absent at the time of transplantation. Improved antibody screening and identification techniques as well as improvements in crossmatch techniques have significantly reduced the incidence of hyperacute and accelerated rejection.
The kidney is most susceptible organ to hyperacute rejection. The liver is relatively resistant.
Acute rejection occurs days to weeks (or months in the case of late acute rejection) post-transplant and may contain a cellular (Acute Cellular Rejection) as well as a humoral (Acute Humoral Rejection) component.
Acute cellular rejection, which is the most common form of rejection, is mediated by recipient T lymphocytes that have been activated directly or indirectly against donor antigens, primarily in the lymphoid tissues of the recipient. The donor dendritic cells enter the circulation and function as antigen-presenting cells (APCs) in the direct pathway. As donor APCs die out or are destroyed, recipient dendritic cells process and present alloantigens to recipient T-cell in the indirect pathway. The alloactivated T cells mediate cellular rejection with release of cytokines.
Compared to other solid organs, the lungs appear to be at a particularly high risk for Acute cellular rejection though the reasons for this are not entirely clear.
Acute humoral rejection is caused by pre-existing donor specific anti-HLA antibodies which are negative at the time of the pre-transplant crossmatch and/or de-novo donor specific antibodies. Diagnosis of Acute antibody mediated rejection involves identification of rapid graft dysfunction, accompanied by the presence of circulating anti-donor HLA antibodies and biopsy evidence of C4d deposition in the peritubular capillaries. In kidney grafts, C4d may also be deposited in the glomeruli though this is variable. Acute antibody mediated rejection is reversible with treatment such as plasmapheresis and intravenous immunoglobulin plus increased immunosuppression (with for example tacrolimus and mycophenolate mofetil). The Anti-CD20 antibody Rituximab is also used in some cases.
Chronic rejection appears as fibrosis and scarring in all transplanted organs. The type of injury is organ specific. In heart transplants, chronic rejection manifests as accelerated coronary artery atherosclerosis. In lungs, it manifests as bronchiolitis obliterans (BOS). In liver, it is characterized by the vanishing bile duct syndrome, the progressive destruction and disappearance of intra-hepatic bile ducts leading to cholestasis in which where bile cannot flow from the liver to the duodenum. In kidney recipients, chronic rejection is called chronic allograft nephropathy and manifests as fibrosis and glomerulopathy.
Some drugs used as part of the immunosuppressive regime, such as cyclosporin and tacrolimus, are known to have a nephrotoxic effect and contribute to chronic rejection.
The role of alloantibodies in chronic rejection is increasingly being recognised. Recent data from the 14th international histocompatibility workshop demonstrated that four year deceased donor kidney allograft survival was 20% less in patients with donor specific antibodies compared to donors with no HLA antibodies. MICA antibodies were also demonstrated in these patients. In kidneys, chronic rejection is characterised by slow progressive loss of renal function with endothelial antibody deposition leading to endothelial injury and glomerular basement membrane duplication characteristic of transplant glomerulopathy.
Accommodation
Immunological reactions of interest in the transplant setting include Tolerance, Accommodation and Rejection.
Tolerance refers to a state of sustained non-immune responsiveness to alloantigens. Tolerance is different from Accommodation, which refers to a state of resistance to immune damage. A state of Tolerance remains a goal for solid organ transplantation as it would allow for the withdrawal of immunosuppressive regimes and their associated toxic effect to grafts.
Accommodation permits patients to maintain grafts even in the presence of Donor Specific Antibodies (DSA). Accommodation was originally identified in ABO blood group incompatible renal transplantation in which the graft survived and functioned normally despite the presence in the patient of high titre ABO blood group antibodies. Proposed mechanisms for Accommodation include the expression in the graft of several protective genes which block the activation of the transcription factor NF-KB, thereby suppressing induction of proinflammatory genes and inhibition of the membrane attack complex thereby disrupting the action of complement.
Immune mechanisms which contribute to graft rejection include acute and chronic alloantibody mediated rejection (AMR) as well as acute and chronic cellular rejection.
Tolerance
The clonal selection theory explains how the adaptive immune system is able to respond to an almost infinite diversity of antigen. One potential option for achieving such diversity would have been for the cells of the adaptive immune system to bear an almost infinite number of different receptors, each capable of recognizing a different feature shared by pathogen. This would however have been limiting in terms of the level of diversity that could be achieved given the finite amount of space on the cell surface. Instead, each cell expresses a single specific receptor, generated by a process of somatic recombination. On binding antigen, the cell is activated and produces progeny, all with the same specificity. This generates a clone of cells and is the basis of the clonal selection theory. The clonal selection mechanism allows each individual to expand clones specific for the antigens to which they have been exposed.
The clonal selection theory not only explains how the adaptive immune system is able to respond to an almost infinite diversity of antigen, it also provides a basis for the mechanisms that are involved in developing self-tolerance. These mechanisms include central tolerance by clonal deletion, peripheral tolerance by deletion and inactivation (anergy) and the action of Suppressor T cells.
Central tolerance by clonal deletion refers to mechanisms of tolerance acting during lymphocyte development in the thymus or bone marrow. Experimental studies show that central tolerance is mostly due to the elimination or inactivation of those T and B cells that strongly recognise self-antigens. These cells are destroyed or inactivated after they have expressed receptors for self-antigens and before they develop into fully immunocompetent lymphocytes.
Peripheral tolerance by deletion and inactivation (anergy) refers to mechanisms acting on mature lymphocytes after they have left the primary lymphoid organs. Not all genes are expressed in the thymus so developing T cells cannot be exposed to all self-antigens. Therefore, additional mechanisms for tolerising self-reactive mature T cells are necessary. Mechanisms of peripheral B cell self-tolerance are also necessary because after stimulation with antigen B cells expand and undergo somatic mutation, generating a population of B cells with new antigen specificities. Some of these cells may be specific for self-antigens.
Sometimes T cells are not deleted but become specifically unresponsive to antigen stimulation (i.e. they do not proliferate). This is called clonal anergy. One of the molecular mechanisms responsible for inducing anergy is signalling via CTLA4, a molecule expressed by activated T cells.
While the principal mechanisms of tolerance to self-antigens are clonal deletion and anergy, suppressor T cells have been proposed as a backup mechanism. Suppressor T cells specific for a given antigen are thought to be able to inactivate other lymphocytes specific for the same antigen. For example, under some circumstances it is possible to induce specific tolerance to allografts which appears to be maintained by suppressor T cells.
Apoptosis
Apoptosis is programmed cell death, a process in which cells die in a controlled way as opposed to cells that die as a result of acute injury. Cells that die as a result of acute injury typically swell and burst. This is known as necrosis. Necrosis results in cells that die spilling their contents over adjacent cells, causing a potentially damaging inflammatory response.
In apoptosis, cells die neatly, without damaging their neighbours. The cells shrink and condense. Their cytoskeleton collapses, their nuclear envelopes disassemble and their nuclear DNA break up into fragments. The cell surfaces are altered, displaying properties that cause the dying cells to be rapidly phagocytosed by other cells or by macrophages before any leakage of their contents occurs. This not only avoids the damaging consequences of cell necrosis but also allows the organic components of the dead cell to be recycled by the cell that ingests it. The average adult human loses between 50 and 70 billion cells each day due to apoptosis
The intracellular machinery responsible for apoptosis depends on a family of caspases that have a cysteine at their active site and cleave their target proteins at specific aspartic acids. Caspases are synthesized in the cell as inactive precursors (Procaspases), which are usually activated by cleavage at aspartic acids by other caspases. Once activated, caspases cleave and activate other procaspases resulting in an amplified proteolytic cascade. Some of the activated caspases then cleave other key proteins in the cell. Some caspases cleave the fibrous meshwork of proteins on the inner surface of the inner nuclear membrane, causing the irreversible breakdown of the nuclear membrane. Other caspases cleave a protein that normally holds a DNA-degrading enzyme (a DNAse) in an inactive form, freeing the DNAse to cut up the DNA in the cell nucleus. In this way, the cell dismantles itself quickly and neatly and its corpse is rapidly taken up and digested by other cells.
Apoptosis of T cells plays a central role in developmental, physiologic and pathologic processes including deletion of T cell clones expressing self-antigens in the thymus, elimination of T cells which are infected with viruses and homeostasis of T cell populations that have expanded following high dose antigen exposures.
Transplantation tolerance can be achieved through several mechanisms, including the action of suppressor cells, the induction of anergy or the deletion of graft-reactive donor T cells. Apoptosis may also represent a mechanism of induction of transplantation tolerance.
In the acute phase of rejection, allografts consistently show evidence of massive apoptosis both of the transplanted organ and graft‐infiltrating T‐cells. Ischaemic and reperfusion injury lead to the production of reactive oxygen species which are directly toxic to cells inducing apoptosis and/or necrosis. In liver transplantation, apoptosis contributes to death of hepatocytes and biliary duct cells. In renal transplantation, apoptosis has been detected in the acute and chronic rejection states. Apoptosis has also been detected in pancreatic transplantation. Apoptosis has been observed in intestinal transplantation. Apoptosis has also been shown to potentially play a role in cardiothoracic transplantation.
Apoptosis contributes to the outcome after organ transplantation, being involved both in graft rejection and in transplantation tolerance.
Role of Dendritic Cells in the Alloimmune Response
Dendritic cells are the most potent of the Antigen Presenting Cells (APC) responsible for priming the immune response. Distributed throughout the tissues of the body, they possess surface pattern recognition receptors that recognize pathogen associated molecular patterns making them capable of initiating an immune response to infection. Two main subsets of dendritic cells have been described, the myeloid dendritic cells (mDC) and plasmacytoid dendritic cells (pDC) with the mDC being the more abundant. These are traditionally believed to derive from myeloid and lymphoid precursors respectively, though more recently both cells lines have been proposed to derive from both cell types of precursors. Dendritic cells are capable of stimulating naive T cells and are therefore known as professional APC’s. More recently, an additional role for dendritic cells as important mediators of peripheral immune tolerance and maintenance of immune homeostasis has been described.
Dendritic cells play a central role in the alloimmune response. After kidney transplantation, donor derived dendritic cells, triggered into maturation by the transplant Ischemic and Reperfusion Injury (IRI) as well as locally released pro-inflammatory cytokines, migrate from the kidney to the recipient lymphoid organs where they stimulate the host immune response through the direct, indirect and semi direct routes of allorecognition. These functions of Dendritic cells set in train the rejection response. Transplanted organs are eventually depleted of donor derived dendritic cells but it has been shown that recipient dendritic cells that infiltrate transplanted organs sustain the alloimmune response after T-cell activation has already occurred.
Dendritic cells are also considered to play a role in tolerance induction though the mechanism is yet to be fully elucidated. Potential mechanisms include promotion of clonal deletion, the induction of T Regs and inhibition of memory T cell responses.
In stem cell transplantation, the number of circulating plasmacytoid and myeloid dendritic cells and their origin, donor or recipient, have been shown to be associated with the initiation of acute Graft versus Host Disease (aGvHD), relapse and graft failure. The recognition of alloantigen presented by residual host dendritic cells to donor T cells in the direct pathway of allorecognition initiates GvHD. Ongoing antigen presentation involves donor derived dendritic cells presenting host antigen to donor T cells in the indirect pathway of allorecognition. Some studies have shown that the absolute numbers of circulating dendritic cells post stem cell transplantation is an independent predictor of aGvHD. Patients with aGvHD after stem cell transplantation have lower numbers of circulating mDC and pDC compared to healthy individuals but do have a higher number of dendritic cells in affected areas such as the skin.
Underlying mechanisms of the Inflammatory Response
Inflammation is the body’s response to insults or injury such as would result from infection, trauma, burns, hypersensitivity to toxins, irritants and allergens etc. The inflammatory response involves a variety of mechanisms to defend against pathogens and to repair tissue damage.
During inflammation, numerous types of inflammatory cells are activated. Each inflammatory cell releases cytokines and mediators to modify activities of other inflammatory cells. Orchestration of these cells and molecules leads to progression of inflammation and the five classical signs of inflammation i.e. heat, pain, redness, swelling and loss of function.
The inflammatory response proceeds as follows:
- The activation of resident cells at the site of infection or injury (mast cells, resident macrophages and dendrite cells) and rapid entry of granulocytes (neutrophils, basophils and eosinophils) in response to injury in the innate immune response
- The innate immune system responds rapidly to infection or injury with macrophages, NK cells, CD8+ T cells and neutrophils providing an early response
- Infiltration of effectors immune cells (lymphocytes) to enable specific immune responses in the adaptive immune response follows
- The adaptive immune response is mediated primarily by CD4+ T cells which are primed by dendritic cells. Dendritic cells and macrophages are antigen-presenting cells, which stimulate naïve T cell proliferation. Originating in bone marrow, dendritic cells reach tissues through blood circulation. Once the dendritic cell identifies, ingests and processes an antigen, it migrates to the lymph nodes and presents the antigen to resident T cells, inducing the immune response
- Recruitment and activation of mesenchymal cells such as endothelial cells and fibroblasts to form new blood vessels and a collagenous matrix
- Tissue remodelling
Inflammation is characterized by the release of chemical mediators leading to vascular changes, primarily vasodilatation and cellular changes.
Amongst the chemical mediators released are histamine, prostaglandin and complement factors. Histamine triggers vasodilation and increases vascular permeability allowing cells to enter tissue at the site of injury. Prostaglandins increase the effects of other substances that promote vascular permeability or affect the aggregation of platelets, which is a part of the clotting process. Activated complement proteins serve as chemotactic factors for neutrophils, increase vascular permeability and stimulate the release of histamine from mast cells.
The initial vascular change is vasoconstriction. This is transient and is followed by vasodilation, under the influence of regulatory molecules on endothelial cells of blood vessels. When blood vessels dilate the walls of the blood vessels become more permeable and allow protein-rich fluid along with water and salts into the tissues of damaged area which is what causes the swelling.
Cellular changes then follow. In less than an hour after injury or infection, a large number of neutrophils reach the site of injury, under the direction of a concentration gradient of chemical mediators. Within 24 to 28 hours of the initiation of the inflammatory process, monocytes which have matured into cell-eating macrophages, reach the site of infection. However, if the inflammation is caused by parasitic worms, eosinophils predominate in the inflammatory response rather than neutrophils.
While the acute inflammation is fundamentally beneficial, severe inflammation can lead to systemic inflammatory response syndrome, which is characterized by hyperinflammation and can cause organ injury, shock and death in its most severe forms.