KIR Matching in HSCT
KIR mismatching in HSCT plays a role in reducing relapse, mortality and GvHD, particularly in the mismatched setting such as in 11/12 unrelated transplants and in haploidentical related transplants. KIR mismatching may also potentially play a role in cord blood transplantation where HLA mismatched transplantation is more common though more studies are required to demonstrate this.
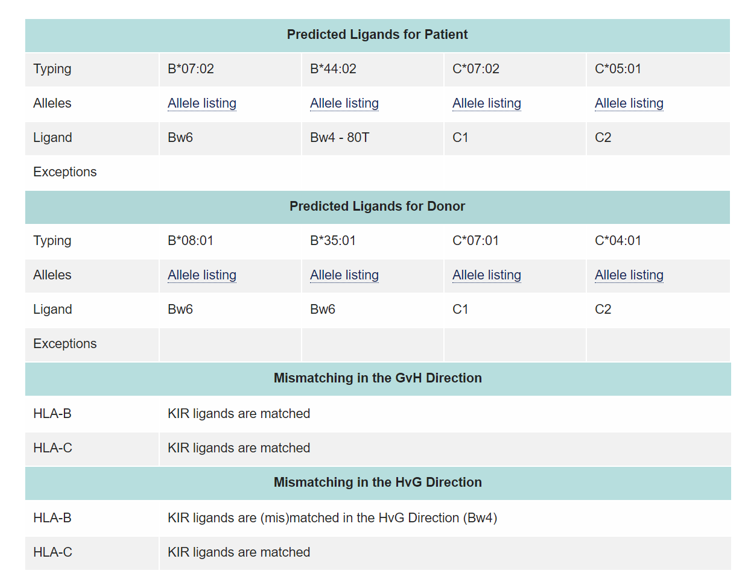
Some studies have shown that KIR ligand compatibility or incompatibility influence the outcome of HSCT. The ligands for KIR receptors are HLA class I molecules. These include HLA-C locus antigens with either Asn (Group 1 HLA-C antigens) or Lys (Group 2 HLA-C antigens) at position 80, the HLA-Bw4 epitope and some HLA-A antigens
The absence of a HLA-ligand in the patient and the presence of the corresponding inhibitory KIR in the donor means that inhibition of the donor NK cell reactivity cannot take place leading to the potential generation of NK alloreactivity against the patient target cells, resulting in alloreactivity in the graft-vs. host (GvH) direction. This is also the main mechanism contributing to the beneficial GvL effect by reducing relapse rates. In addition, this also contributes to a decrease in aGvHD risk by destroying recipient antigen presenting cells (APCs) and consequently to an improvement in OS. Conversely, the presence of a HLA ligand for KIR in the patient and its absence in the donor will result in alloreactivity in the rejection (HvG) direction.
The role of mismatches of the KIR antigens themselves, as opposed to mismatches of the KIR ligands, has not been extensively studied.
The KIR ligand calculator at https://www.ebi.ac.uk/ipd/kir/ligand.html can be used to calculate the KIR ligand GvH vs HvG mismatch.
Relevance of MICA and MICB
One study has shown that in a series of well-matched HLA-A, B, C, DRB1, DQB1 stem cell transplants, a higher rate of grades II – IV acute GvHD was observed in patients mismatched for MICA compared to those matched for MICA. The MICA mismatched patients had more gastrointestinal aGvHD than the matched patients, possibly reflecting the tissue distribution of MIC antigens.
Minor Histocompatibility Antigens
HLA presents the major genetic barrier to stem cell transplantation. However, evidence that other genetic systems are involved includes GvHD and some degree of rejection even when transplanting with HLA identical siblings. A non-HLA system which is thought to contribute to this is the minor histocompatibility antigen (MiHA) system. Minor histocompatibility antigens comprise of peptides derived from proteins in which some degree of polymorphism exists such that there may be differences between the patient and donor repertoires. These peptides can be presented to the immune system by both HLA class I and II antigens. Minor histocompatibility antigens are target for cytotoxic T lymphocytes (CTL’s) and mismatching them can lead to GvHD and to rejection depending on the direction of the mismatch.
The best characterised minor antigens are the Y chromosome derived HY peptide and the autosomal HA1 to HA5 peptides. Minor histocompatibility antigens such as HA1 and HA2 have restricted tissue distribution and are present normally only on haematopoietic cells. Others such as HY are more ubiquitously distributed, expressed for instance on gut epithelium. HA1 and HA2 are expressed on leukemic cells and some tumour cells, making them potential targets for cellular therapy. In mice, allogeneic stem cell transplantation donor CD8+ T cells specific for a MiHA found in the recipient has been shown to inhibit the division of leukemic cells. However, there is a risk in developing GVHD if the T cells are specific for MiHAs expressed ubiquitously on epithelial cells. Immune cell restricted MiHAs such as MiHA HB-1, are ideal targets for graft-versus- leukemia (GVL) since not all nucleated cells would be targeted by responding T cells.
Minor HLA antigens are restricted by certain HLA types. HA1 and HA2 for instance are presented by HLA-A2 and HY is presented by multiple class I and II HLA antigens.
Cytokines
The other non HLA genes thought to play a role in haematopoietic progenitor cell donor selection and transplant outcome are Cytokine genes. The belief that a variable ability to produce pro-inflammatory and other cytokines as a result of polymorphisms in the promoter and regions of these cytokine genes has lead to many studies attempting to understand the role of cytokine polymorphisms in HSCT. Unfortunately, these studies have not lead to a consensus view of the role of cytokine genes in transplant outcome and therefore in donor selection. There is some tentative evidence that transplantation with donors who express genes that make them high producers of TNFα and IL-1 leads to increased GvHD. Levels of cytokine production may also have an influence on susceptibility to infection post-transplant.